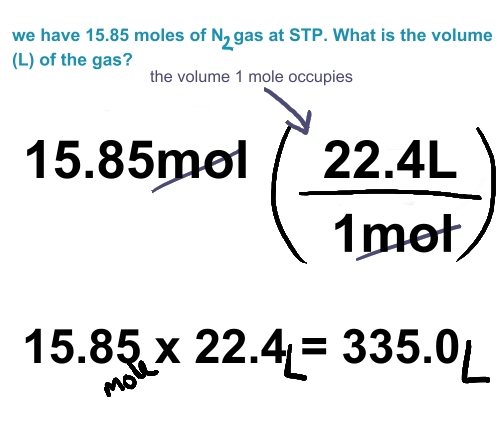
1) At 0 degree C and 101.3 kPa: 1 mol= 22.4 L
- This temperature and pressure is called STp
- 22.4 L/mol is the molar volume at STP
Example:
How many litres will 4.7 mol of O2 have at STP?
4.5 mol x 22.4 L = 1.0 x 10^2 litres
1 mol

http://www.youtube.com/watch?v=AHqNiEwcXiE
- Candace Chan
No comments:
Post a Comment