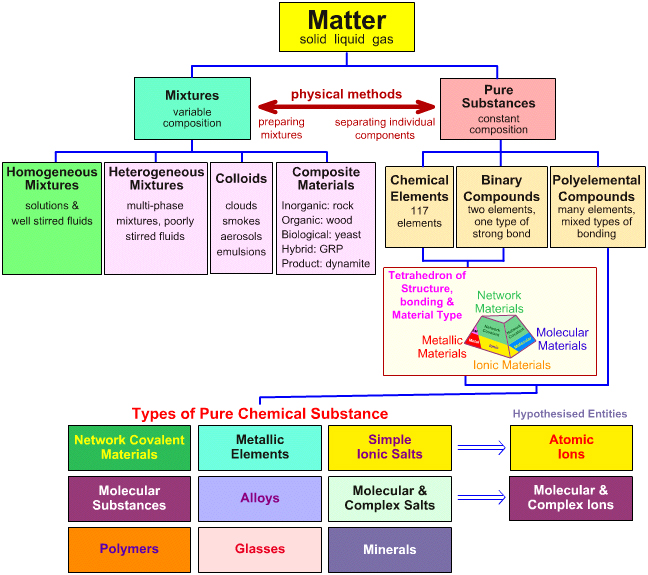
-Matters can be classified into Homogeneous substances & Heterogeneous substances
-Homogeneous: consists of only 1 visible component
-e.g. water, oxygen, graphite, air, brass
-Heterogeneous: contains more than 1 visible component
-e.g. granite, chocolate chip cookie, sand
/ Solution
/ Homo. Mixtures
/ Homogeneous / Element (e.g. oxygen, Iron)
Matter \ Pure Substances
\ Heterogeneous \ Compound (e.g. water, sugar)
\ Mechanical Mixtures
p.s. Solution - 2 or more substances
- usually involves liquid (e.g. fog, steel)
- component in greater amount=solvent
- water is the most common solvent
- (aq) is used when sth is dissolved in water
- component in smaller amount=solute
Seperating Mixtures
-Many methods
- By hand
- Filtration --> Physical
- Distillation --> Changes
- Crystallization
- Chromatography
By Stan K.