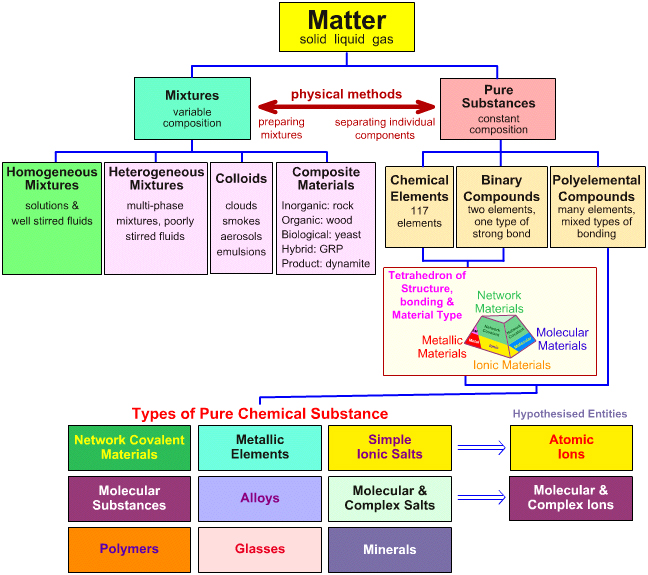
Metric system
Giga 10(9) centi 10(-2)
Mega 10(6) milli 10(-3)
Kilo 10(3) micro 10(-6)
Hecto 10(2) Nano 10(-9)
Deca 10(1) Pico 10(-12)
Femto 10(-15)
-gigabyte and megabyte = computers
-error is inescapable because of inaccurate measurements.
Meniscus= a curved surface of a liquid.
Error might occur in an experiment because of:
-inaccurate measurements
-measuring always involves estimation.
Measuring instruments are never completely free of flaws.
Absolute error= (measurement – accepted)
Absolute percent= (measurement – accepted %) x100
Accepted
-Reo